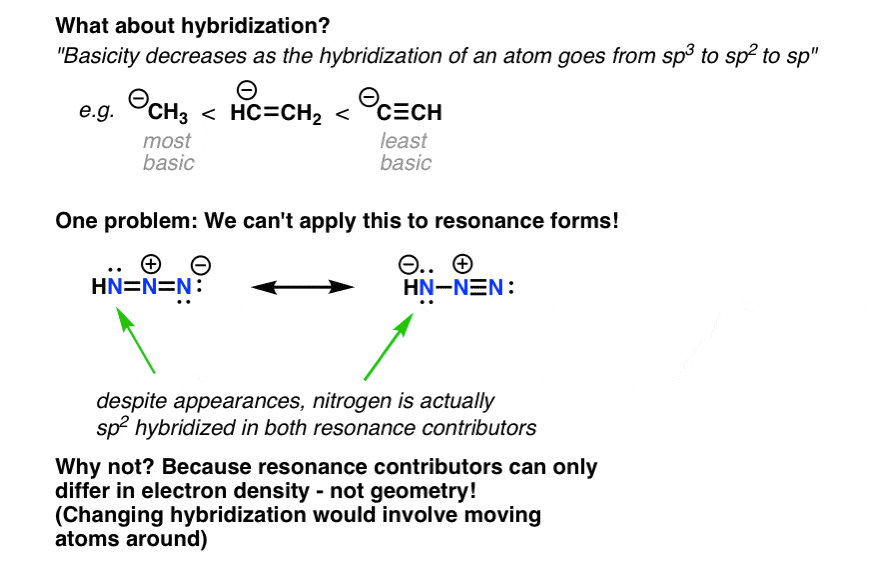
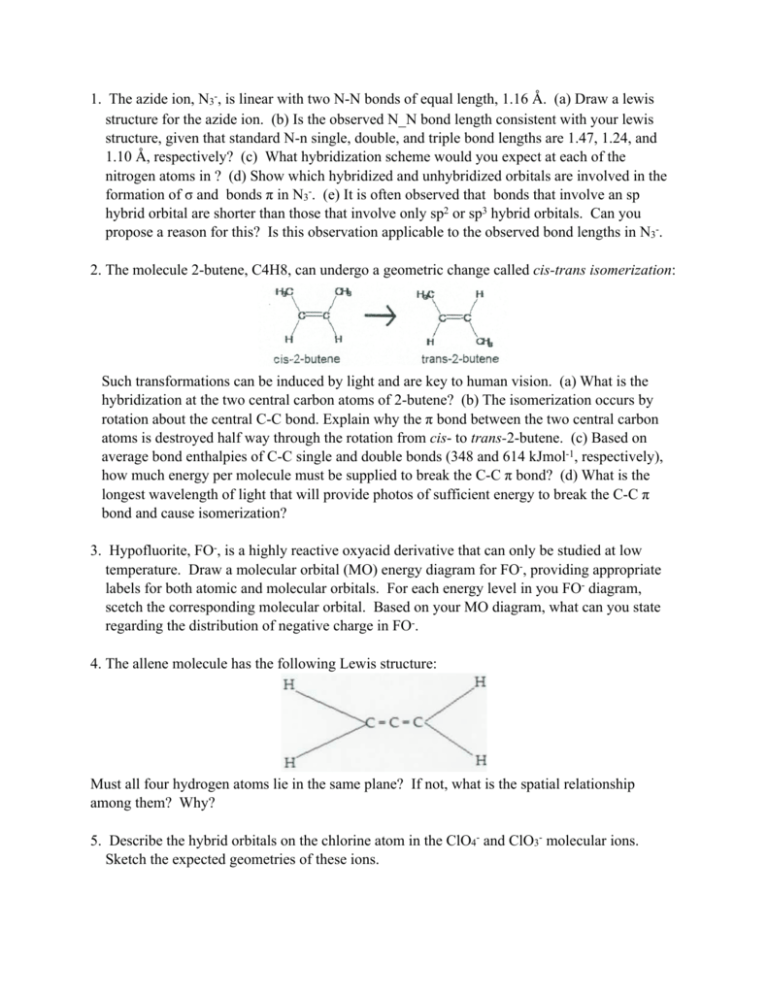
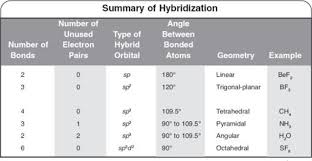
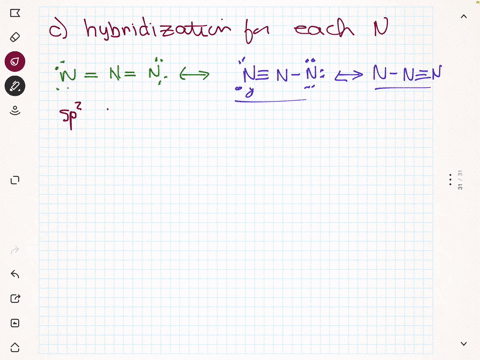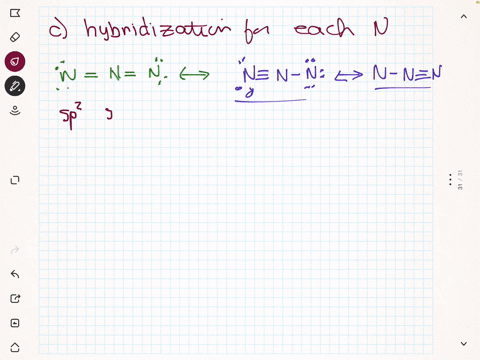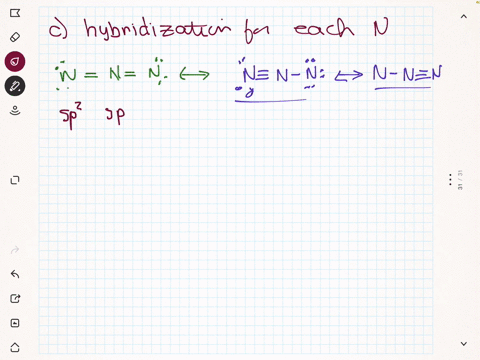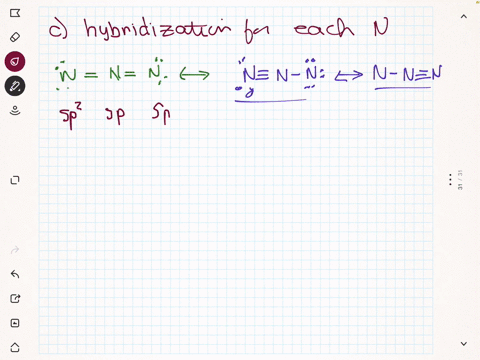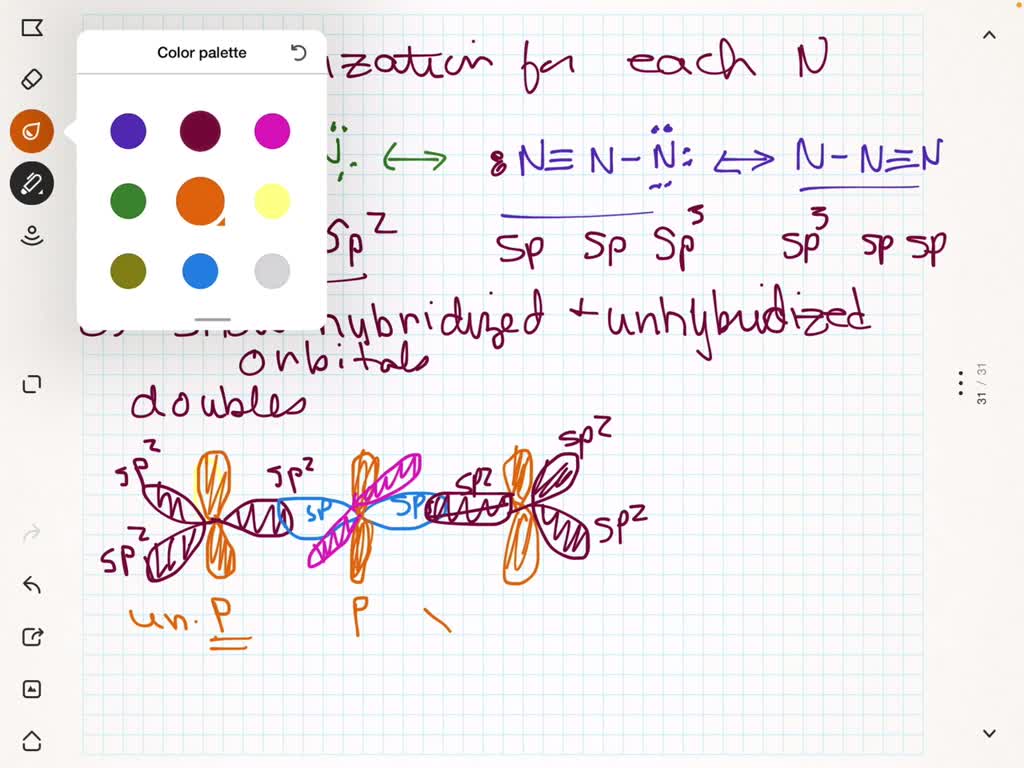The azide ion (N_3) is a linear triatomic molecule. Determine the representations for the s and p orbitals on the central atom. | Homework.Study.com
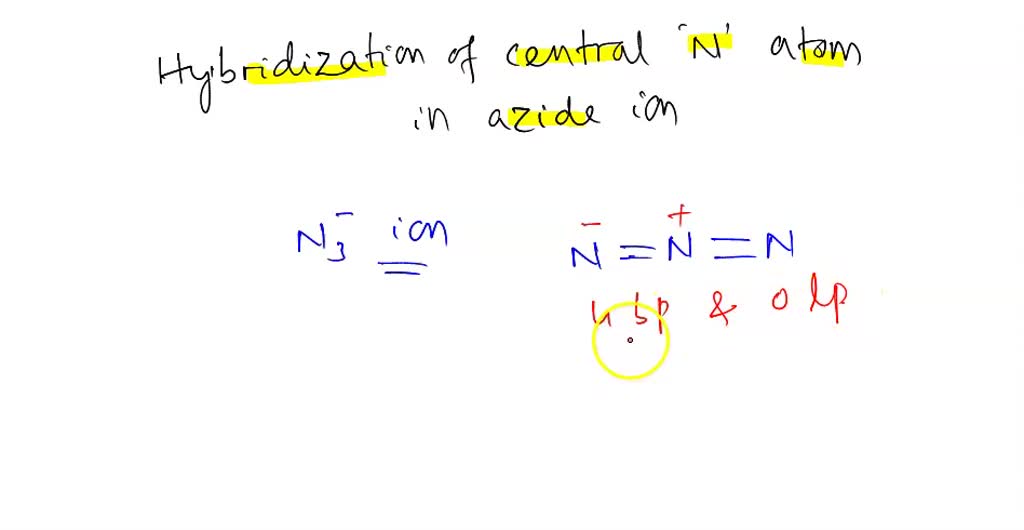
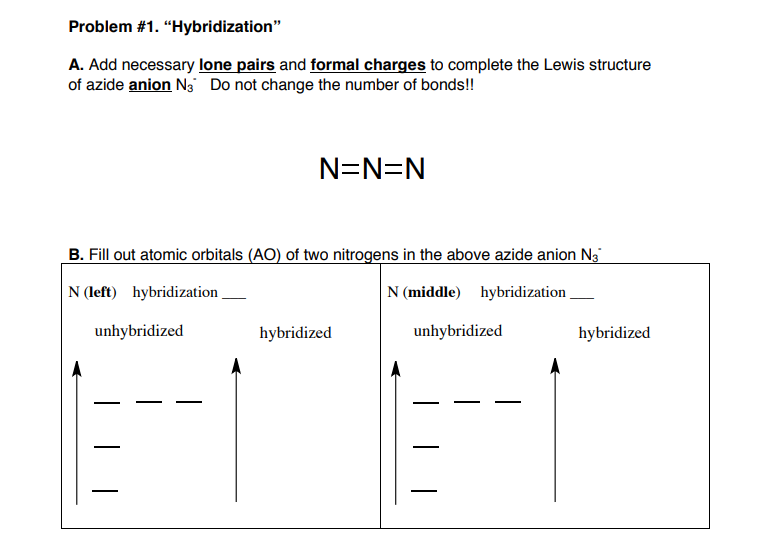
SOLVED: Select the single best answer: What is the hybridization state On the central N atom in the azide ion, N; ? sp Sp Sp d sp?

The hybridisation of central atoms in {N_3}^-, NOCl and N_2O respectively are:sp^2,, sp^2,, spsp,, sp^2,, spsp,, sp,, sp^3sp^2,, sp,, sp

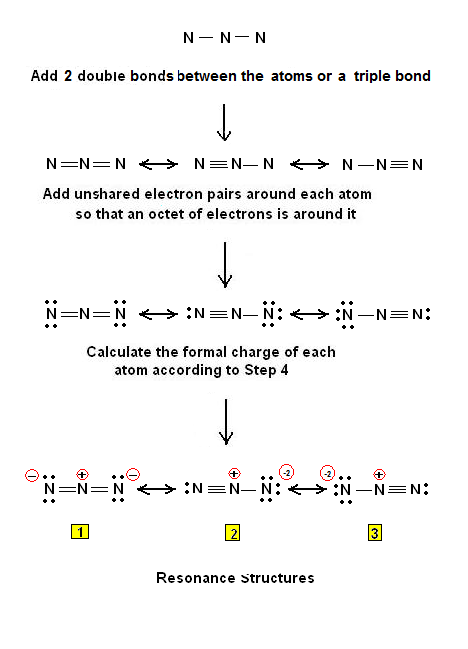
N3- lewis structure, molecular geometry, hybridization, bond angle | Molecular geometry, Molecular, Electron configuration

What is the hybridization of nitrogen in the azide ion ? | 12 | CHEMICAL BONDING & MOLECULAR ST... - YouTube

What is the hybridization of nitrogen in the azide ion ? | 12 | CHEMICAL BONDING & MOLECULAR ST... - YouTube